- Подготовка производственной площадки к маркировке
- Сроки внедрения маркировки для производителей: что нужно знать малым, средним и крупным?
- Оборудование для маркировки: какое нужно и что пригодится из того, что у вас уже есть?
- Состав кода маркировки молочной продукции
Release 4.42 MDLP 20 November 2021
Information about the content of the release in Russian can be found at this link.
Installed on Sandbox test environment: on 09/11/2021
Period of installation on the production environment: on 20/11/2021
XSD-schemes version: 1.37.
Improvements
Set of schemes 1.37
Within release, version of xsd schemes has been increased to 1.37.
Updating of the schemes set will extend the functional possibilities of MDLP FGIS business processes.
Set of schemes 1.37 and updated business processes are backward compatible with the current version of schemes 1.36. A feature for submitting the schemes of ver. 1.34, 1.35 and 1.36 remains.
Description of the changes can be found at link.
Movement of the medicines with “Awaiting introduction into circulation by owner” status
Medicines with “waiting_for_release” (“Awaiting introduction into circulation by owner”) status can be moved under operation 431 - “Registration in the MDLP IS of information on movement of the medicines between different business places”.
Business place/responsible storage management by participants
Activity status of the business place/responsible storage can be managed in the interface of the MDLP FGIS in “Registry of business place” and “Registry of responsible storage” sections. Therefore, the possible accepted values of parameters in the “Status” column have been renamed:
- “Valid” → “Active”;
- “Invalid” → “Blocked”.
Goods circulation participant can independently control activity of own business place/responsible storage. This may be necessary in implementing operations to remove business place/responsible storage and for further prohibition of the operations with the relevant business place/responsible storage to the remaining goods circulation participants. Description of the operations available for blocked business place/responsible storage can be found at link.
New parameter “Acts under license” was added into “Registry of business place” and “Registry of responsible storage” with the following possible values:
- “Valid”;
- “Invalid”.
This parameter is set automatically by the MDLP FGIS and reflects the activity status of the business place/responsible storage under the license received from Roszdravnadzor.
Report on remains of imported medicines before introduction into civil circulation
“Remains of imported medicines before introduction into civil circulation” report has been implemented for issuers of the marking codes. This report is generated for the marking codes with the following emission types:
- “Foreign manufacturing”;
- “Marked in the customs control area”.
The report relies on SGTIN in the following statuses:
- “Awaiting shipment to the Russian Federation”;
- “Transferred for disposal”;
- “Shipped to the Russian Federation”;
- “Awaiting confirmation of return at the time of importation”;
- “Imported to the Russian Federation”;
- “Awaiting ownership change confirmation”;
- “Declared”;
- “Admitted to the warehouse from the customs-controlled area”;
- “Marked in the customs-controlled area”;
- “Awaiting confirmation by importer”.
The report is in the section “Reports” → “Remains of imported medicines before introduction into civil circulation” (Figure 1).
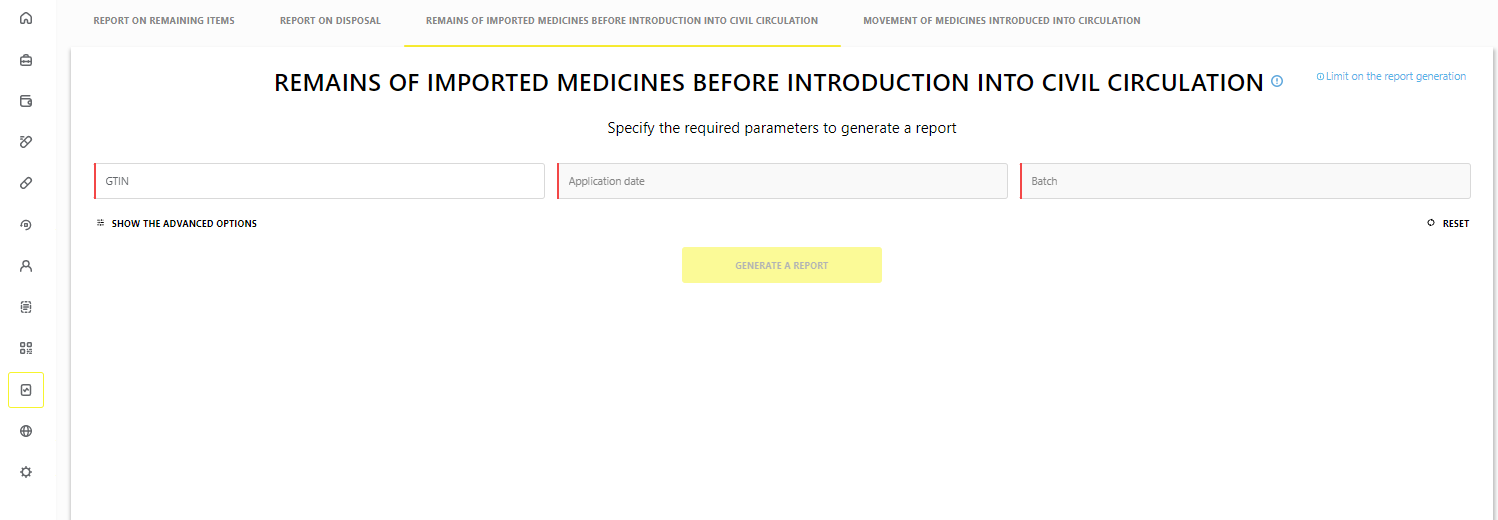
The following rights are required to work with the report:
- “VIEW_BATCH_REPORT” (“Viewing the reports on the medicines”);
- “MANAGE_EXPORT_TASKS” (“Asynchronous unloading management”).
The report can be unloaded:
- through the interface of the MDLP FGIS in *.csv format;
- via API by means of asynchronous unloading.
Detailed information on the report is available in the information statement. To access the information statement on import movement report generation, click icon right from the report name.
Movement of medicines introduced into circulation (available from 23/11/21)
The report has been implemented for issuers of the marking codes. The report contains information on change of owners of the medicines if the change of the owners of the medicines introduced into circulation has been recorded. The report takes into account the following operations:
- 415 - Medicine shipment from warehouse;
- 416 - Medicine acceptance to warehouse;
- 441 - Shipment of the medicines to unregistered business place;
- 461 - Medicine import from the Russian Federation to EEU;
- 471 - Handover to a new owner;
- 472 - Medicine shipment under agency contract;
- 473 - Medicine acceptance under agency contract;
- 701 - Shipment / acceptance confirmation;
- 702 - Posting.
The report is in the section “Reports” → “Movement of medicines introduced into circulation” (Figure 2).
The following rights are required to work with the report:
- “VIEW_LP_MOVEMENT_CIRCULATION_REPORT” - “Viewing of the report on movement of the medicines introduced into circulation”;
- “MANAGE_EXPORT_TASKS” - “Asynchronous unloading management”.
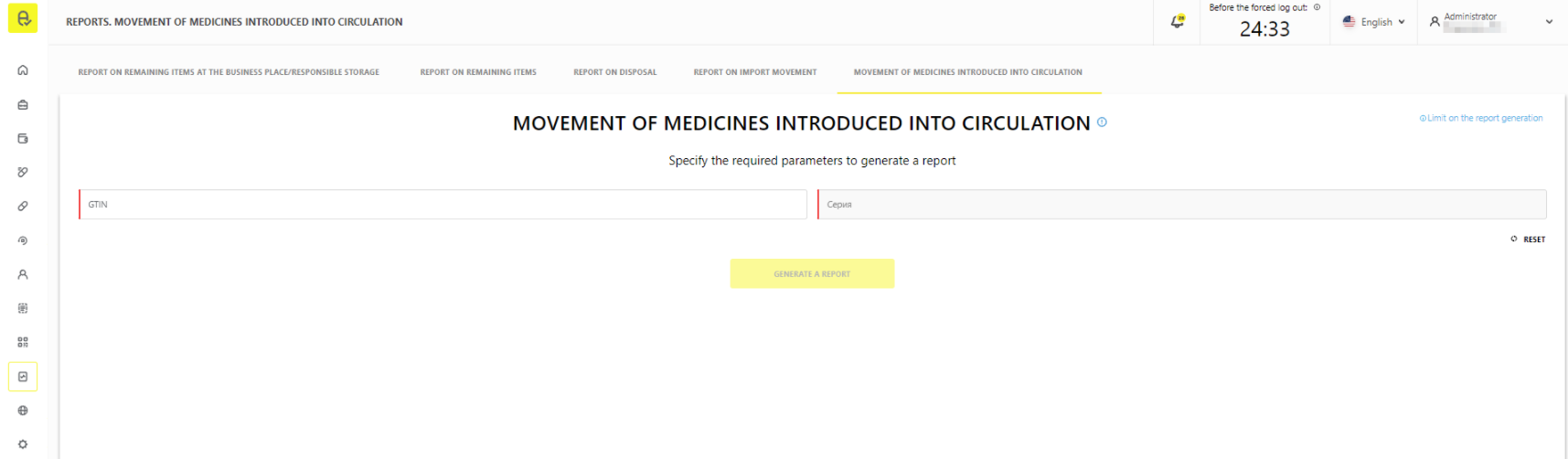
The report can be unloaded:
- through the interface of the MDLP FGIS in *.csv format;
- via API by means of asynchronous unloading.
Detailed information on the report is available in the information statement. To access the information statement on generation of the report “Movement of medicines introduced into circulation”, click icon right from the report name.
Report on remaining items at the business place/responsible storage
The following statuses have been added into the rules for generating the report on remaining items at the business place/responsible storage:
- “Awaiting confirmation of return”;
- “Awaiting confirmation of suspended medicines return”;
- “Transferred for disposal”;
- “Shipped to EEU”;
- “Shipped to unregistered business place”;
- “Circulation suspended”;
- “Withdrawn from circulation”.
Notification management
Possibility to manage the user notifications has been added into the user account interface. This functionality can be found in the section “Administration” → “Notification management”.
If “MANAGE_ACCOUNTS” (“User Account management”) right is available, management of the notifications of all active users of the respective organization will be available in this section. Receipt of the notification is connected with a corresponding right. If user does not have a certain right, it will not be possible to activate notification receipt until the right is granted.
The required right will be indicated in the pop-up message when you click on the information message “Insufficient rights” against the notification name (Figure 3).
Accessible information channels:
- e-mail;
- MDLP FGIS notification center.
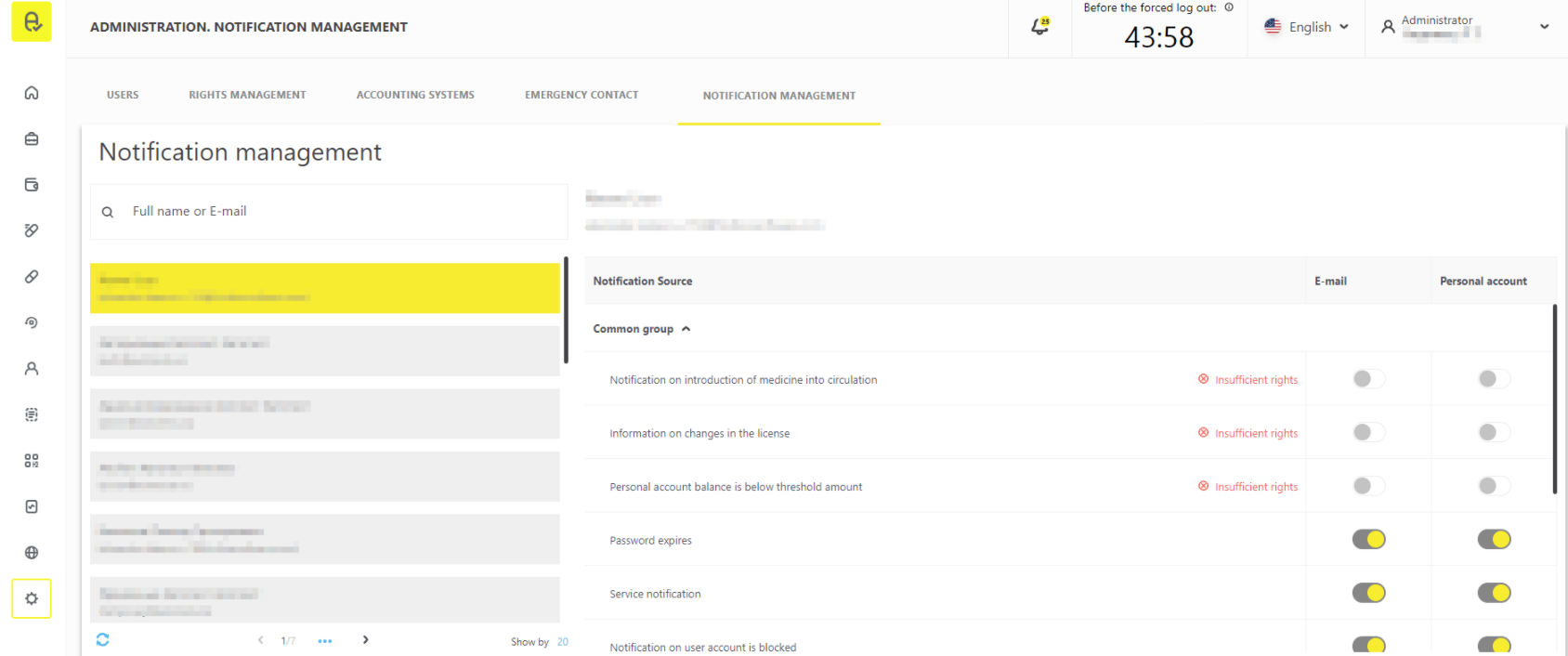
Please note that settings for user notification have been moved from the section “Profile” → “Notification settings” to the section “Administration” → “Notification management”. No special rights are required for the management of own notifications.
Synchronization of license information
“Synchronize information” button was added into “Pharmaceutical licenses/medical licenses/licenses of circulation of narcotic drugs” and “Production licenses” sections.
Clicking on this button will send a request for synchronization of information on existing licenses of the goods circulation participant.
A request in each section can be sent 1 time per 24 hours.
To get the request results, user should independently check the modified list of the licenses in the “Pharmaceutical licenses/medical licenses/licenses of circulation of narcotic drugs” and “Production licenses” sections.
General improvements
- The following were added into the sections “Products” → “SGTIN registry” and “Products” → “Registry of SGTINs emitted before 2021-03-28”:
- “Total withdrawn proportion”, “Total written-off proportion”, “INN of the organization that conducted the selling (online marketplace)” parameters;
- status “Partially written-off”;
- withdrawal type “Sold by distance selling”.
3. A new system notification was implemented when restoring user access.
4. The validity period of the code for restoring the password has been changed from 10 days to 24 hours.
5. “Search” button has been renamed “Search on a page” in the registries of the MDLP FGIS interface.
API modifications
More information on revision list can be found in documentation. To receive technical documents on API methods, please contact technical support at support@crpt.ru.


