- Подготовка производственной площадки к маркировке
- Сроки внедрения маркировки для производителей: что нужно знать малым, средним и крупным?
- Оборудование для маркировки: какое нужно и что пригодится из того, что у вас уже есть?
- Состав кода маркировки молочной продукции
Release 4.38.3 MDLP on April 16, 2021
Information about the content of the release in Russian can be found at this link.
Installation on the "Sandbox" test environment: 16/04/2021
Installation on the production environment: 16/04/2021
Improvements
“Report on remaining medicines” is implemented for manufacturers
Codes issuers (manufacturers and marketing authorization holders (hereinafter the MA)) will have access to information on medicines location.
All data in the report are grouped as follows:
- by organizations with medicines on a balance at the time of report generation;
- by addresses of the business places up to the locality level.
Remaining items are calculated by SGTIN.
The report is available for viewing in the interface of the user account on the “Report on remaining medicines” tab in the “Reports” section with the possibility of export to Excel (Figure 1). Export is available after filtering with “GTIN”+”Batch” parameters.
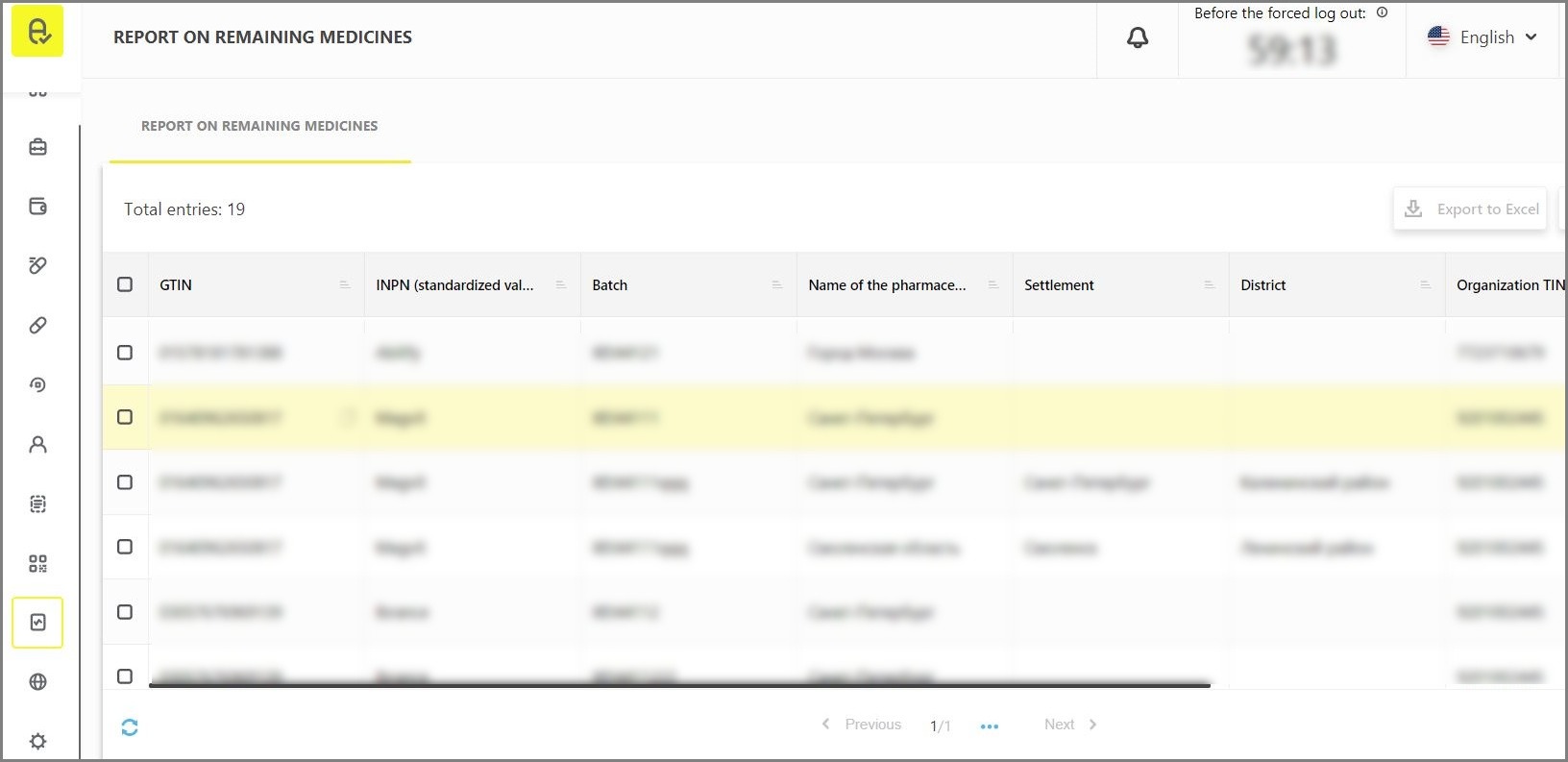
Please note that the reports section is available to all participants by default, but the availability of data in the report depends on the availability of codes emitted by Participant or on the availability of delegated access to specific GTIN/batches.
Feature of delegation of access to data for reporting has been implemented
As part of new report viewing features, it is possible to delegate access to other participants for certain GTIN or specific batches. Reports can be formed on the basis of these data.
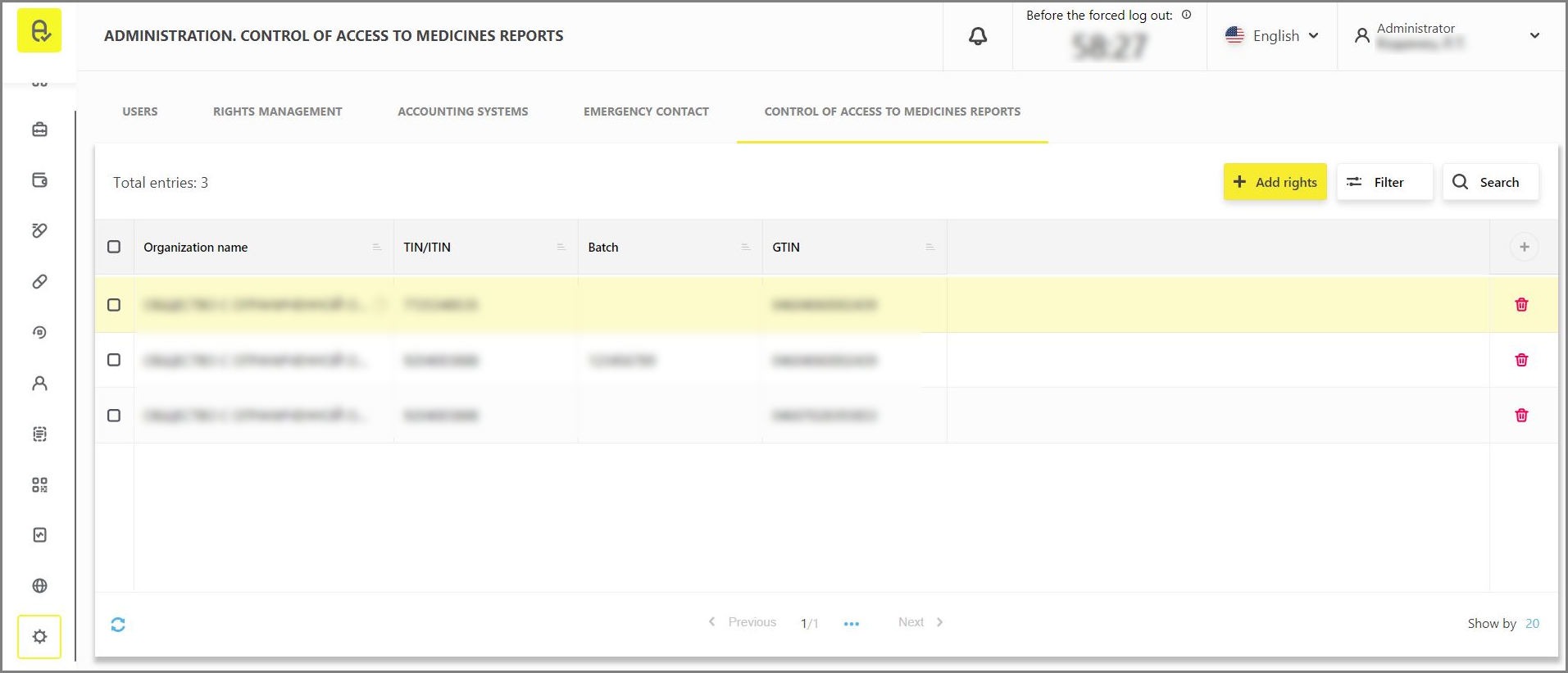
Delegation features can be found on “Control of access to medicines reports” tab in “Administration” section and is available only to codes issuers (manufacturers or MA holders).
To grant a new right, click the "Add rights" button and follow the system hints (Figure 2):
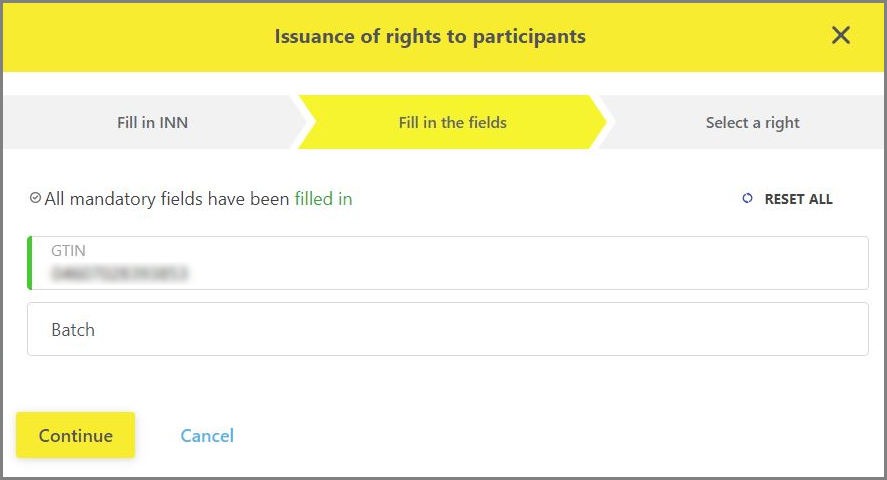
- As a first step, it is necessary to enter INN/ITIN of the organization of Participant who needs to be granted the viewing right (Figure 3);
- Next you should specify GTIN or GTIN and batch (Figure 4);
- In the last step, the system searches for the Participant and checks the possibility of transferring rights for the specified information. If everything is entered correctly, you will be asked to click the "Save сhanges" button to activate the transfer of viewing rights (Figure 5).


In case if it is necessary to revoke the previously granted right to view, it is sufficient to click the "Delete” button in the rightmost cell of the table and confirm the action in the modal window.
Please note that a Participant who has delegated access to data to another organization also retains the possibility to view reports on these data.


