- Подготовка производственной площадки к маркировке
- Сроки внедрения маркировки для производителей: что нужно знать малым, средним и крупным?
- Оборудование для маркировки: какое нужно и что пригодится из того, что у вас уже есть?
- Состав кода маркировки молочной продукции
Release 4.35 MDLP on November 19, 2020
Information about the content of the release in Russian can be found at this link.
Installed on Sandbox test environment: 19.11.2020
Installed on Sandbox test environment: 21.11.2020
XSD-schemes version: 1.36
Improvements
Switch of system operation to a set of schemes 1.36
All changes are backwards compatible.
The possibility of submitting schemes of versions 1.34 and 1.35 is saved.
A detailed description of the changes can be found in the document "Composition of changes for Participants with release of the set of schemes 1.36" published on the official website.
A new process for recording information on the acceptance of medicines by the agent under the agency contract has been implemented.
To support this process, scheme "473 – agent_acceptance” has been developed, as well as notification "624–agent_acceptance_notification" sent to the consignor after scheme 473 has been processed.
A new process for recording information on the logic return of medicines imported previously into the Russian Federation has been implemented. To support this process, scheme “337–return_import" has been developed, as well as notification "625–return_import_notification" sent to the consignor after scheme 337 has been processed.
Please note! It is necessary to contact the CRPT technical support service for providing access to the scheme 337.The process of medicine selling in the retail trade using CRE (including the release of medicine on subsidy prescription with partial payment) has been finalized. The possibility of the medicine release on the documents other than cashier's cheque has been implemented. Scheme "512-withdrawal_without_kkt" has been developed to support this process;
The process of correction of previously sent information has been finalized. The possibility to adjust the type of production order has been ensured. Changes have been introduced into scheme "253-change_information".
New scheme "812–successor_acceptance" - "Registration in MDLP System of information about medicinal drugs moving to warehouse of successor" has been developed.
Currently, the scheme is disabled, and included into xsd-schemes set for initial consideration.Changes were made to SGTIN limit (= 30000) in the schemes:
- 10311–Registration of information on packing stage completion in MDLP IS (information from SKZKM);
- 10319–Registration in MDLP IS of information on medicine package and application of identification means outside of the Russian Federation (information from SKZKM).
The following schemes were deleted:
- 311 – Registration in MDLP IS of information on packing stage completion;
- 321 – Registration in MDLP IS of information on finished product release outside of the Russian Federation;
- 811 – Registration in MDLP IS of information on repacking and re-marking of medicines.
New types of financing sources were added:
- Funds from the budget of extra-budgetary funds;
- Combined budget funds.
General improvements
New statuses have been added for SGTIN
Registry of products by SGTIN supports the display of new statuses and provides the ability to filter them:
- Waiting for confirmation of a foreign medicine return;
- Issued under the documents;
- Partically issued under the documents.
The mechanism of SGTINs automatic removal for Schemes 702 and 703 has been updated
During the processing of schemes 702 and 703, the SGTIN is checked for the level of nesting into the shipping package.
- If the scheme contains SSCC (boxes) which are aggregated into another shipping package (pallet), the boxes are automatically removed from the pallet.
- If SGTINs are specified in the scheme and it is found that the codes are aggregated into the shipping package (box), which in turn is also aggregated into another shipping package (pallet), the mechanism of automatic removal of SGTIN from the box will be activated, as well as the automatic removal of all the boxes from the pallet.
As a result of these actions, SGTIN is no longer aggregated into the box, pallet is disaggregated.
Sending of information to Roszdravnadzor AIS, after scheme 703 is processed, has been added
After scheme 703 is processed, the medicine specified in the scheme shall be checked. If the medicines have been marked in one of the EEU countries, information on the medicine import will be sent to Roszdravnadzor AIS.
The possibility of switching to SGTIN card has been added to "Registry of products by SGTIN"
Switch to "View card by SGTIN" button (Figure 1)
The card displays information about the last operation performed with this SGTIN, as well as the history of successfully accepted documents in which it was used, except for aggregation and transformation operations (Figure 2).
From the card you can switch to the document registry by pressing the Identifier of the particular document.
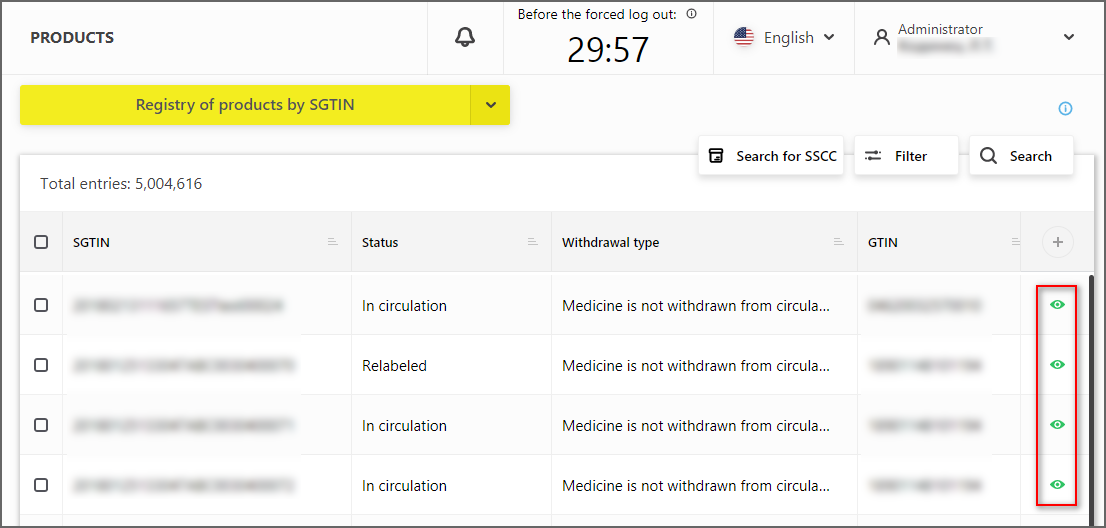
Figure 1
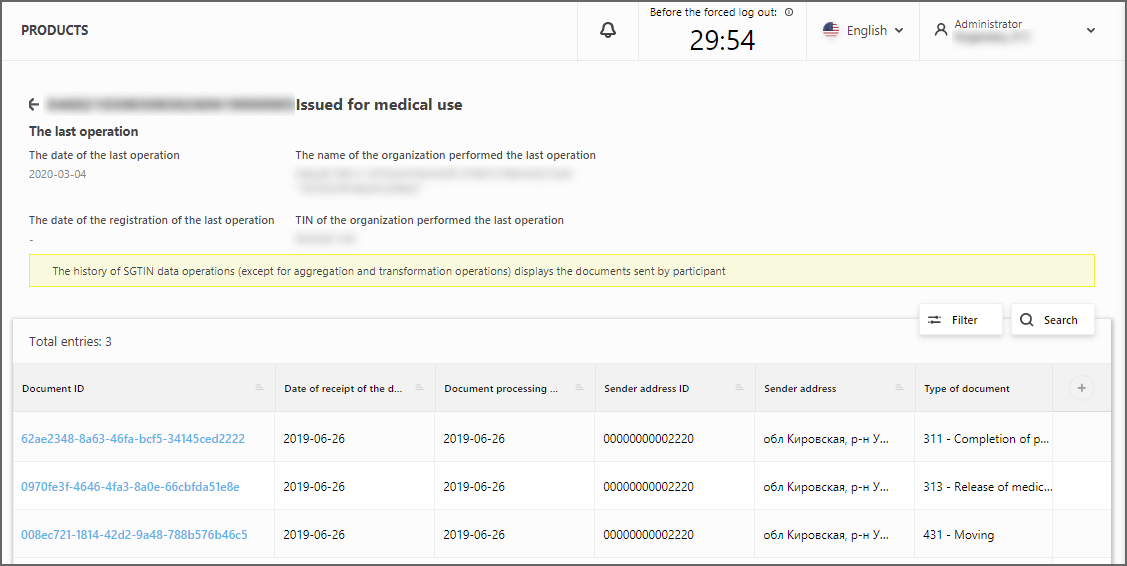
Figure 2
Changes in the document registry
- New types of outcoming documents have been added:
1.1. 337 - Logical return of medicines imported to the Russian Federation;
1.2. 473 - Acceptance of medicines under agent contract;
1.3. 512 - Dispensing of medicine other than indicated in the register receipt. - New types of incoming documents have been added:
2.1. 625 - Notification on logical return of medicines imported to the Russian Federation;
2.2. 624 - Notification on acceptance of medicines under agency contract. - For greater ease and expediency of searching the connection of the document with the notification for the registry of outcoming and incoming documents, the field "Notification Connection Identifier" (with the filtering on it) has been added. Knowing this identifier, you can find the document and the notification that was formed on its basis. Thus, Participants can assist each other in finding the necessary documents if the need arises.
- The field "Document Number" (with the possibility of filtering on it) is added for outcoming and incoming documents. The field is filled in from the "doc_num" attribute if it is present in the document.
New functionality has been added in the "Medicines" section
In the medicine registry, the feature of filling in new optional attributes - "FEACN code" and "Manufacturer Contact" (Figure 3) has been added.
When specifying the FEACN code, it is necessary to enter the code that is used in the customs declarations filled out when these medicines are imported into the Russian Federation. It is highly desirable to fill in this field for those Participants who import medicines into the territory of the Russian Federation.
When specifying the manufacturer’s contact details, it is necessary to enter e-mail and the phone of the organization that can help other Participants in case of any questions regarding these medicines occur.
Important! Do not specify the personal data of the employees of the organizations, use only public, non-personalized information.When filling in new attributes, it is necessary to remember that filling is done at once for all medicines with the same marketing authorization number (Figure 4).
In addition:
- The medicine registry can be filtered by MA number.
- New fields are added in the medicine registry:
2.1. Completeness;
2.2. Primary quantity in consumer packaging;
2.3. Primary package weight/volume;
2.4. Country of MAH;
2.5. FEACN code;
2.6. Manufacturer contact;
2.7. Previous marketing authorization number;
2.8. Validity period of the previous marketing authorization;
2.9. Name of previous MAH. - A new "Additional Attributes" block has been added to a card for the detailed data on a specific medicine, which displays the new "FEACN code" and "Manufacturer contact" fields.
- In “General registry of registered medicine” the following fields have been added:
4.1. FEACN code;
4.2. Manufacturer contact;
4.3. Completeness.
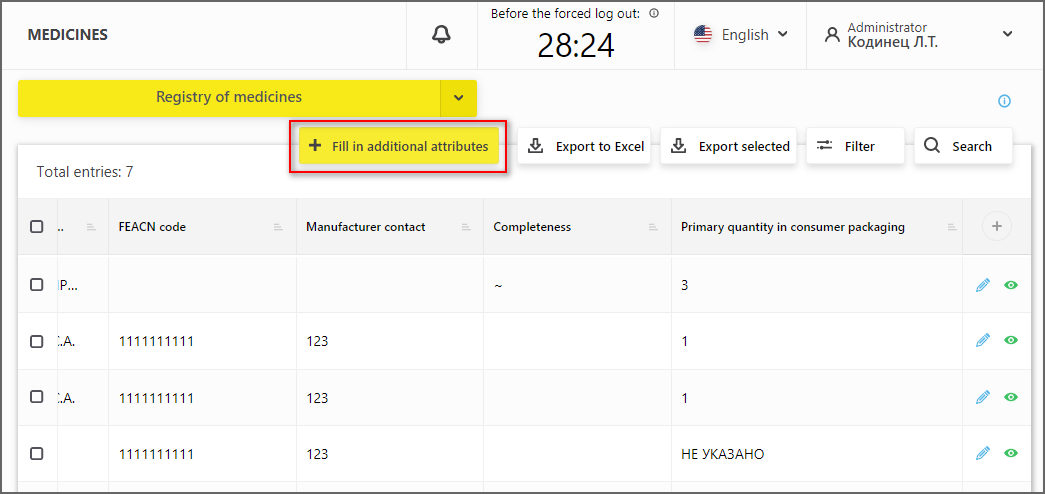
Figure 3
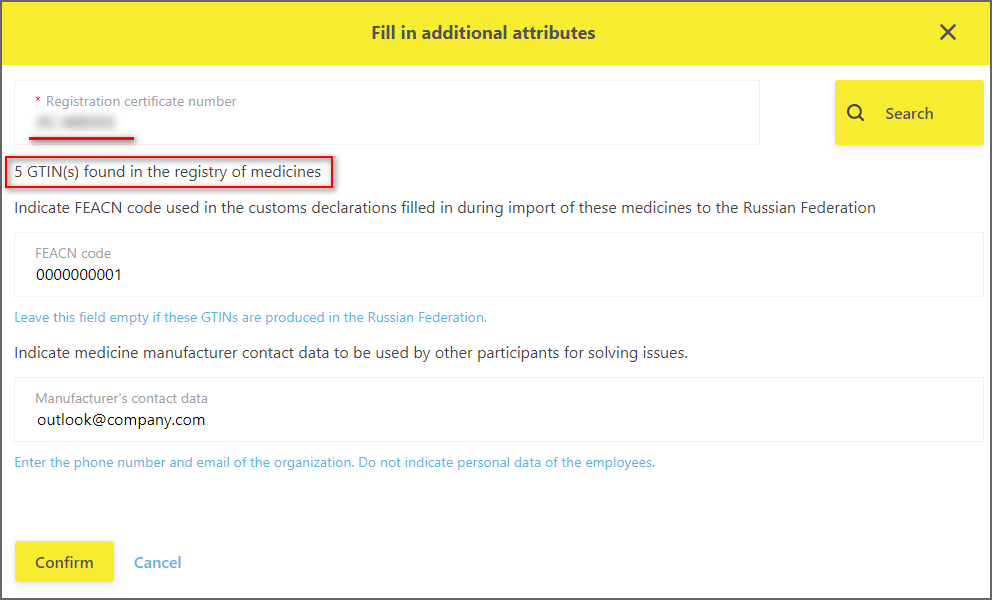
Figure 4
Computer local time check has been added
If an incorrect local time is set on the computer that is used to open the Participant’s personal user account, the timer (to display the time until the end of the session) will not be displayed. The user will see a notification instead.
Notification display features have been added
In the upper right corner, a new “Bell” element (Figure 5) is added next to the timer to display the time until the end of the session. If there are unread notifications, their number will be displayed next to the bell. Clicking on the "View All Notifications" button opens a separate page on which the list will display previously received notifications (Figure 6).
With the addition of a new element, the receiving of the following notifications has been automated:
- " The notice on the certificate expiration". Only for participants – the residents of the Russian Federation. When the certificate expiration date will approach, the system will remind its holder of this event;
- "The notice on getting the information of introduction of medicine into civil circulation". The system will notify the Participant when the information on the introduction of the medicine into civil circulation is received from Roszdravnadzor;
- "The notice on getting the information on license register changes". Upon receipt of information from AIS Roszdravnadzor on changes in the Participant’s licenses, a new notice will be formed.
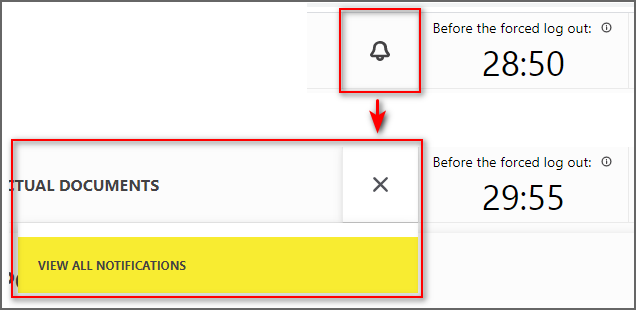
Figure 5
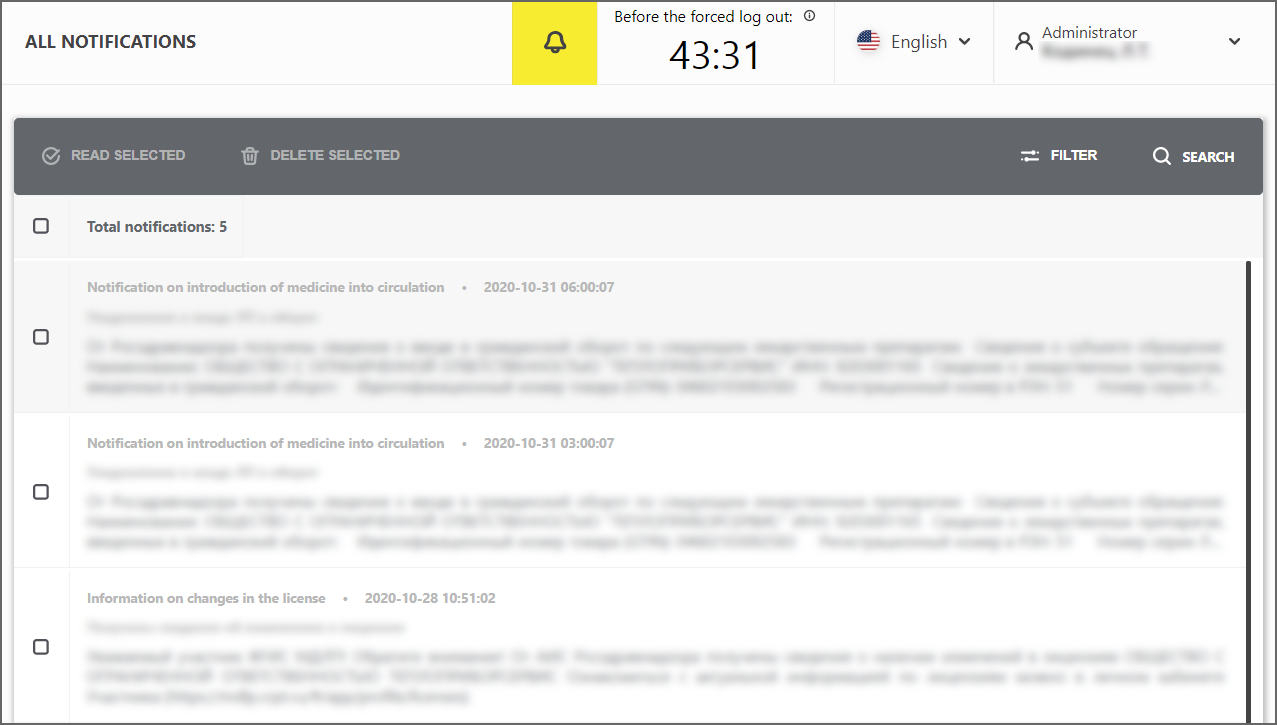
Figure 6
In the "Notifications Settings" tab of the "Profile" section, the feature of enabling/disabling of certain types of notifications is added. It is also possible to specify the method of receiving notifications, either by e-mail or in the user account interface (Figure 7).
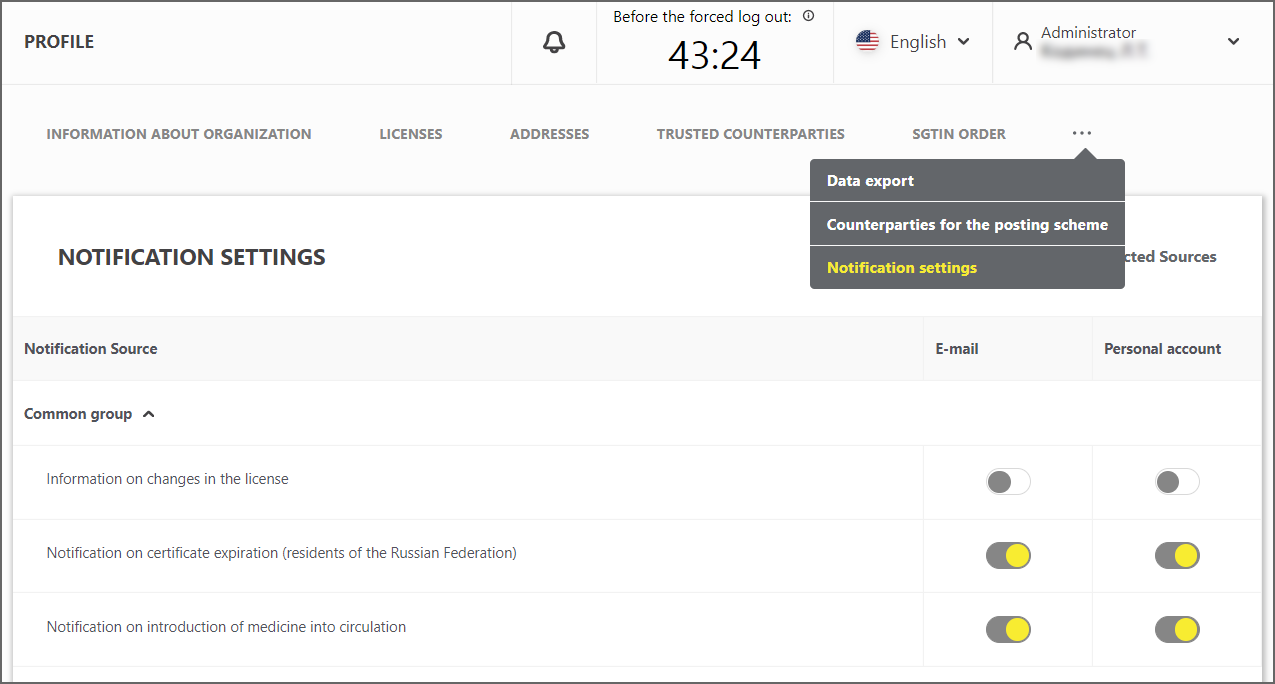
Figure 7
In the “Contractual documents” section, the new types of documents are supported now.
- "Information letter" – does not require to be signed by Participant;
- "Operator’s Notification" – does not require to be signed by Participant;
- "Certificate of Property Loss". First, the Participant signs, then the Operator-CRPT. The Certificate is signed by the parties upon the loss of the disposal registrar.
These document types will be displayed in the "Contractual documents" tab. As in the past, the Participant will receive e-mail notification of the availability of new documents.
In the section "Finance", a switch to the "Financial Documents" tab was added
The tab allows you to view and download the closing documents sent to the Participant after the closing of the reporting periods (Figure 8).
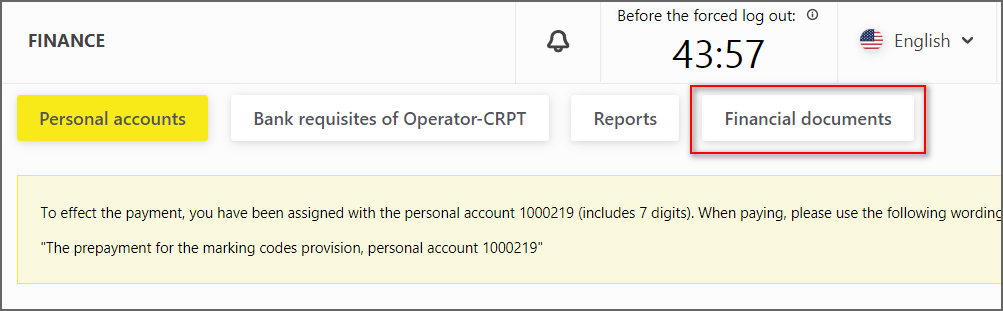
Figure 8
Feature to create the DR malfunction report has been added
"Send DR malfunction report" button (Figure 9) has been added in the disposal registrar registry in front of each registrar.
By pressing the button, the form to create reports of the new type - "DR malfunction report" is opened (Figure 10).
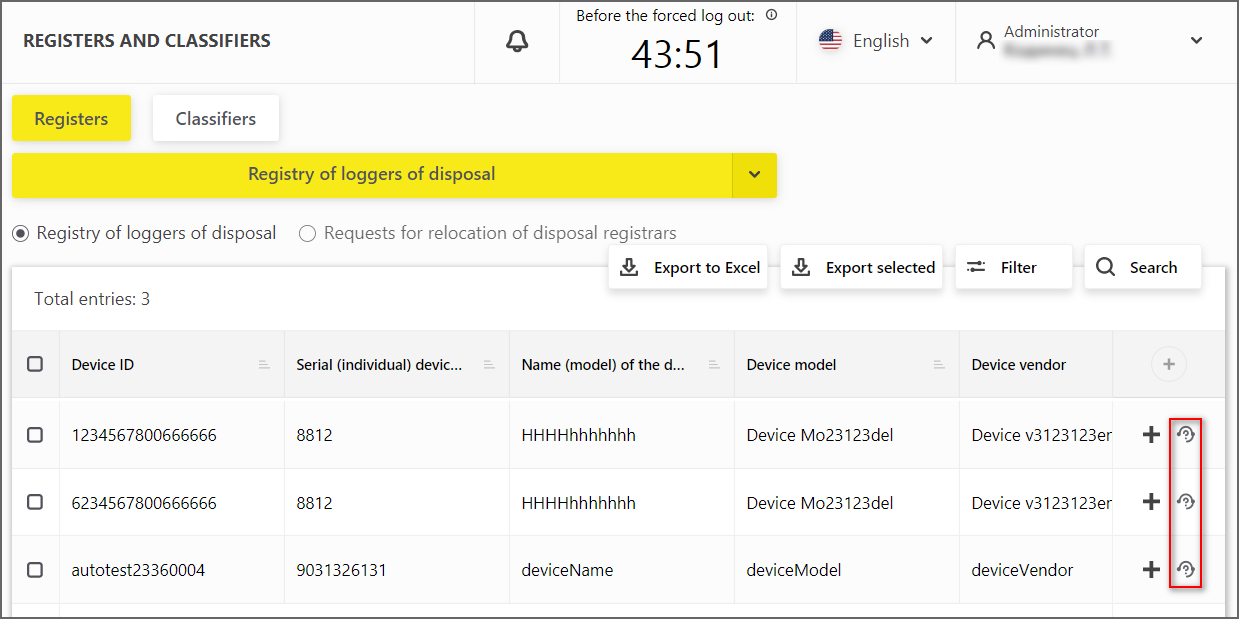
Figure 9
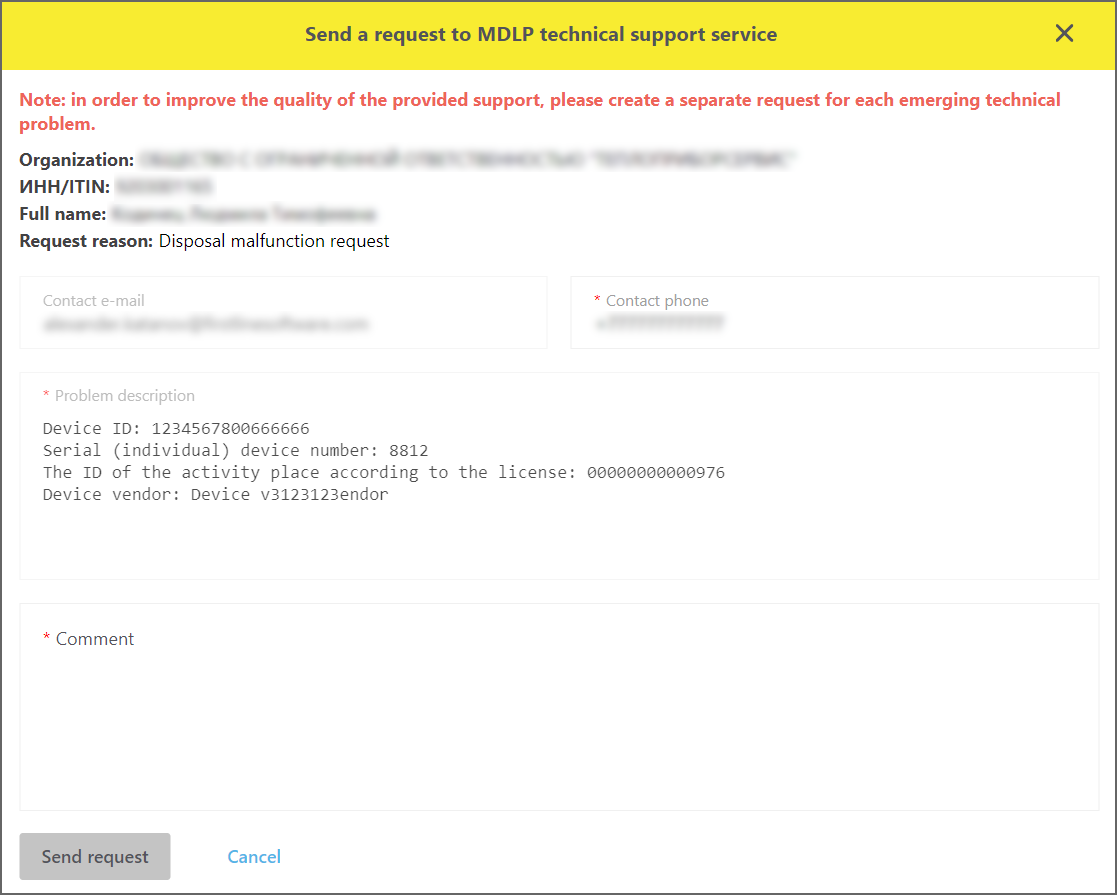
Figure 10
A tool for forced sending of batch data to Roszdravnadzor AIS has been developed
Using this tool, a Participant may re-send medicine data to Roszdravnadzor AIS in cases where an error occurs during the automated information transmission and there is no data in Roszdravnadzor AIS or the batch volume differs from the one declared in MDLP.
To use the tool, it is necessary to:
1. Open the section "Clarification of information in Roszdravnadzor AIS" and switch to the required tab, depending on the country in which the medicine has been produced (Figure 11).
For medicine produced in the Russian Federation or imported from the EAEU, it is necessary to specify:
- GTIN;
- Batch No.;
- Batch volume.
For medicine imported into the Russian Federation (foreign production), it is necessary to specify:
- Trade declaration number;
- Declaration registration date;
- GTIN;
- Batch No.;
- Batch volume.
2. The data shall then be sent for verification (Figure 11);
3. If the data correctness verification is completed, the data can be sent to Roszdravnadzor (Figure 12).
This if the way the data on several batches can be sent at once.
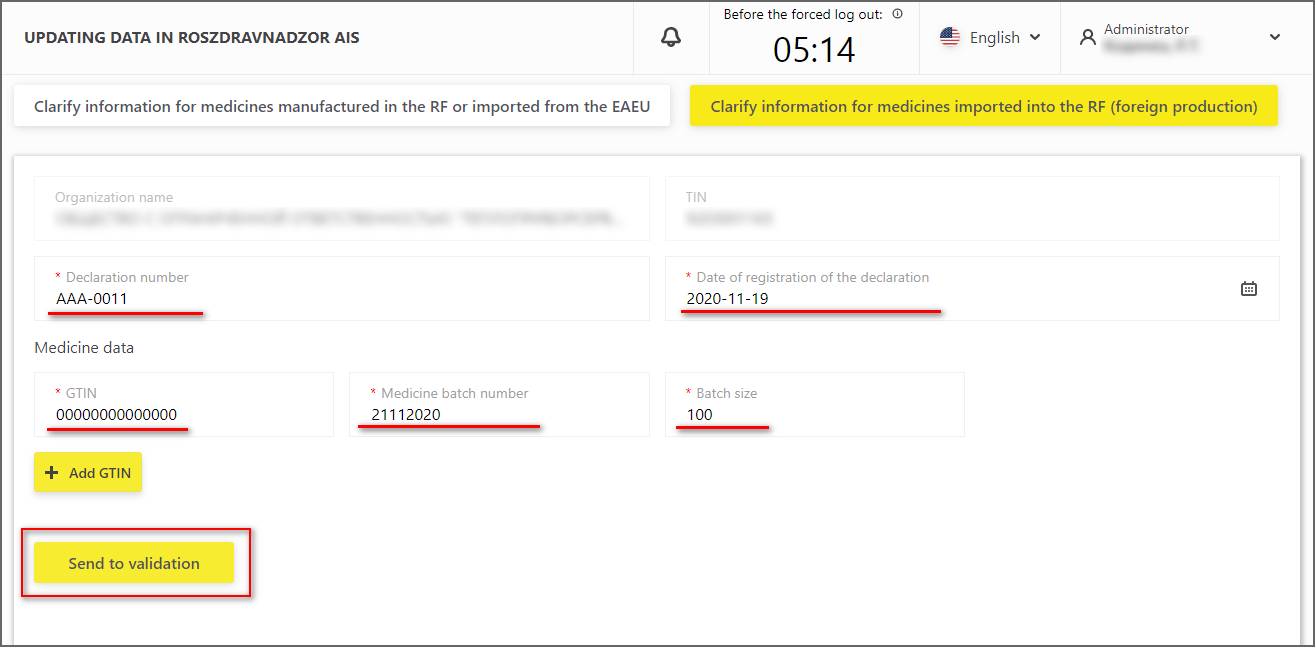
Figure 11
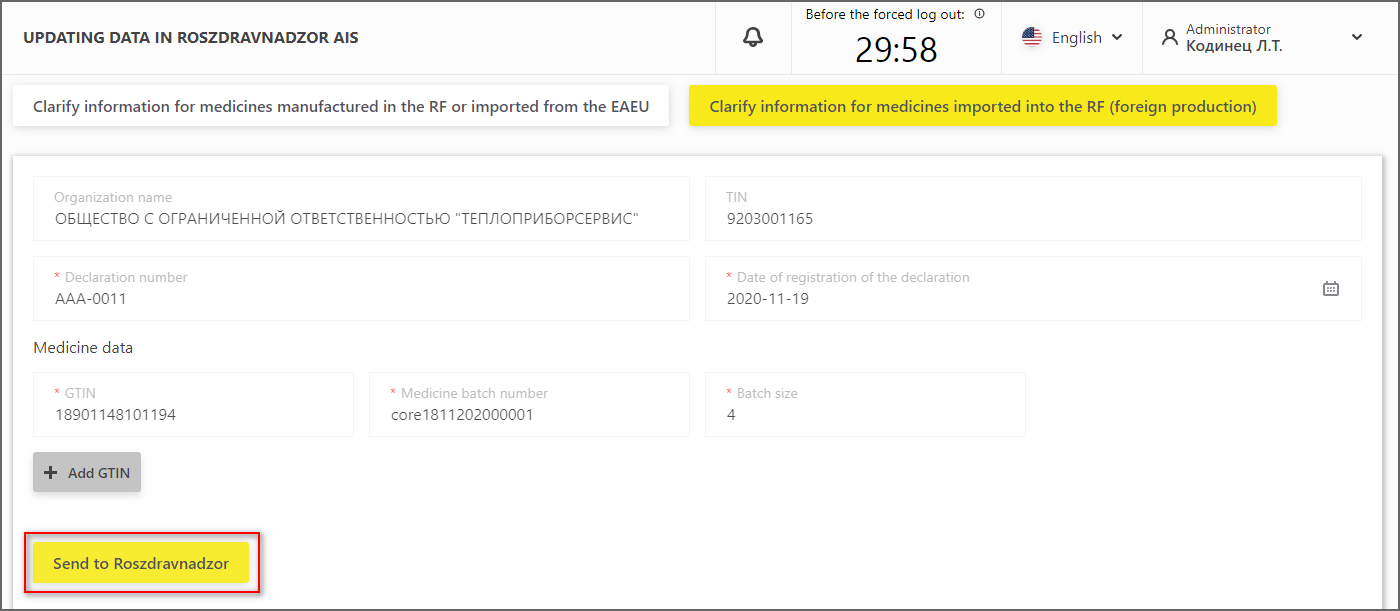
Figure 12
API improvements
More information on revision list can be found in documentation. To receive technical documents on API methods, please contact technical support at support@crpt.ru


